Urea and ammonium nitrate: differences in the use of fertilizers, when it is better to fertilize with one, and when with another
Obviously, you got to this article, because you cannot decide which of the two fertilizers to choose, or you want to understand which one is best suited for your specific case. So, indeed, urea (carbamide) and ammonium nitrate are the two most popular mineral nitrogen fertilizers, but they are by no means the same (in a chemical and biological sense). Accordingly, each of them has its own peculiarities of application, advantages and disadvantages.
Note! In some sources, you can find a rather entertaining remark that urea is an organic fertilizer ... However, urea, like ammonium nitrate, is nitrogen fertilizers, in other words, it is chemical compounds.

Content
- 1 What are the differences between the use of urea and ammonium nitrate: the main points
- 1.1 Granule color
- 1.2 Nitrogen concentration
- 1.3 Nitrogen form
- 1.4 How best to use: methods of application
- 1.5 When it starts to act: which is faster, which is slower
- 1.6 What time of year to apply to the soil
- 1.7 At what temperature is it better to use
- 1.8 How it changes the acidity of the soil: acidifies or alkalizes
- 1.9 What can and cannot be mixed with
- 1.10 How to improve the effect of fertilizers
- 1.11 Do these fertilizers cause the accumulation of nitrates in plants?
- 1.12 Use for spring and autumn eradication spraying (treatment)
What are the differences between the use of urea and ammonium nitrate: the main points
By the way! The site already has separate articles about the features, rules and areas of application urea and ammonium nitrate.
Granule color
- Urea granules almost always pure white color... Have ammonium nitrate they are also white, but usually with a yellow tint, they also say "turn yellow" (in very rare cases it happens the other way around).
Nitrogen concentration
- Carbamide (urea) - the most concentrated nitrogen fertilizer - 46% nitrogen.

- Content nitrogen in ammonium nitrate — 33-35% (average 34.4%).

However! It should be understood that plants do not get all the nitrogen that is present in the listed fertilizers.
Experts have calculated the average utilization rate nitrogen plant from fertilizers (KIU). So, for urea it is 0.5, and for ammonium nitrate - 0.7. Accordingly, when using urea, about 23% of nitrogen is actually absorbed by the plant, and 24.08% of ammonium nitrate.
Nitrogen form
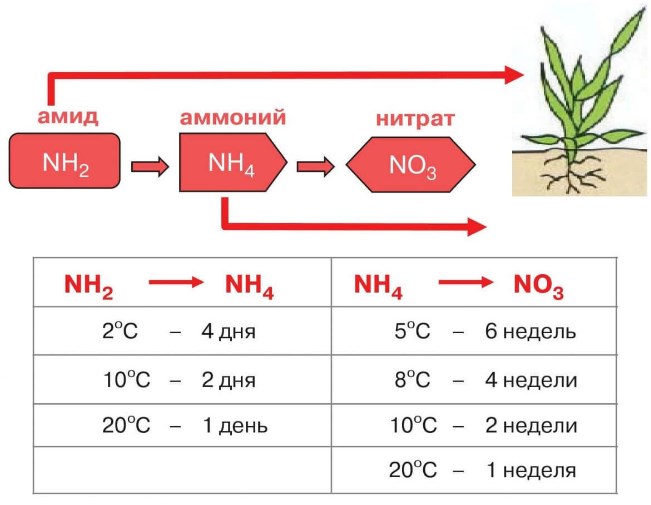
The explanation for the difference in the initial content and the final availability of nitrogen for plants lies in the differences in nitrogen forms, which behave differently after entering the soil.
- So, in urea nitrogen contains in amide form... Chemical formula - (NH2)2CO, carbonic acid diamide.
- In ammonium nitrate, nitrogen there is in two forms at once - ammonium (ammonia) and nitrate ... Chemical formula - NH4NO3, ammonium salt of nitric acid (ammonium nitrate).
Video: conversion in soil and loss of nitrogen when using carbamide (urea) and ammonium nitrate
How best to use: methods of application
- Nitrogen in amide form is absorbed most easily (fastest) through the leaves. Respectively, urea is ideal for foliar feeding (spraying on the leaves)... Moreover, urea solution (up to 1%) does not burn the leaves... Of course, urea can also be applied for digging (to prepare the beds), and as a liquid top dressing.
Important! Urea you need to make (close up) exactly into dry soilto reduce nitrogen losses.
- Ammonium nitrate always apply either in the preparation of the beds in the soil or in the hole, also in liquid form (solution). However, overly concentrated solution (especially in hot weather) can burn the leaves, therefore, ammonium nitrate is almost not used for foliar feeding (spraying over the leaves).
Granules ammonium nitrate can make as into wet soil, so dry.
- Urea granules are needed promptly embed in soil to reduce the loss of ammonia (nitrogen), because hequickly evaporates in the air.
Note! With the surface application of urea, in other words, if you just scatter urea granules without embedding in the soil, then in the absence of precipitation (without irrigation), part of the nitrogen in the form of ammonia simply evaporates. Moreover, this is especially pronounced on soils with a neutral and alkaline reaction.
- Ammonium nitratenot necessarily embedded in soilsince nitrogen in ammonium and nitrate forms does not volatilize in air.
However! It is obvious that the incorporation of granules into the soil (as a rule, it is recommended to a depth of 10-12 cm) will be much more effective than surface application.
When it starts to act: which is faster, which is slower
- Urea is relative slow-acting fertilizer... To make nitrogen in amide form available for consumption by plant roots (for root feeding, application to the soil), a whole chain of chemical and biological transformations must take place (nitrogen from the amide form must go into the ammonium form and then into the nitrate form). This takes time and a relatively high positive temperature (the higher, the faster).
- Accordingly, urea can in some sense be called a sustained-release fertilizer.
However! When applied to the leaves, urea begins to act rather quickly (within 48 hours), which is why it is optimal to use it for foliar feeding (spraying on the leaves).
- Ammonium nitrate - enoughfast-acting fertilizer that is easily absorbed by plantssince contains nitrogen in nitrate (the most rapidly assimilated form) and ammonium form (longer than in nitrate, but faster than in amide).
What time of year to apply to the soil
- Urea add only in spring and during the growing season.
- Ammonium nitrate allowed to make into the soil in autumn, but better in spring and during the growing season.
As we have already found out, ammonium nitrate contains nitrogen in ammonium and nitrate forms.
As you know, ammonium fertilizers are almost never washed out of the soil. But nitrate nitrogen is washed out much easier, especially with heavy precipitation (frequent rains) and on light soils (respectively, it is better to apply in spring).
At what temperature is it better to use
- Urea does not work at low positive temperatures... The optimum temperature for use is above +10 degrees (ideally above +20).
- Ammonium nitrate can be use at low positive temperatures (+5 degrees)... Therefore, it is often scattered over the snow (melting).
It's easy to remember! When it's cold and in the snow introducedammonium nitrate, after get warmer — urea.
How it changes the acidity of the soil: acidifies or alkalizes
- Despite the fact that urea - this physiologically acidic fertilizer, however, after the assimilation of nitrogen by plants, neither acidic nor alkaline residues remain in the soil, in other words, the fertilizer has neutral impact on soil.
- Accordingly, urea can and should even be used to neutralize acidic soils... However, if you have overly alkaline soilthen it is not recommended to use urea.
Let's repeat ourselves! On soils with a neutral and alkaline reaction, urea granules must be embedded in the soil in order to reduce nitrogen losses, otherwise most of it will evaporate.
- Ammonium nitrate -physiologically acidic fertilizer (chemically neutral), but itonly temporarily and locally acidifies the soil, and even with its systematic use (especially for gray and chernozems: on such soils there will be no acidification at all).
However! On acidic soils, slight acidification still occurs with frequent use. Therefore, to neutralize the acidifying effect, together with ammonium nitrate, it is recommended add chalk, lime or dolomite flour, i.e. soil deoxidizers.
Or use calcium ammonium nitrate (already deoxidized fertilizer with neutral acidity).
- Respectively, ammonium nitrate is great for overly alkaline soils in order to increase their acidity (to shift the acidity of the soil to a more neutral reaction).
What can and cannot be mixed with
- Ammonium nitrate is not recommended mix (give at the same time) with alkaline (lime) fertilizers (soil deoxidizers), i.e. with chalk, lime, dolomite flour, wood ash. The fact is that when they interact, a chemical reaction occurs, which leads loss of ammonia (nitrogen) and highlightingunpleasant odorwhich in turn can provokeammonia plant poisoning = toxic shock(especially important for closed ground - greenhouses).
- Urea also do not mix (add at the same time) with alkaline soil deoxidizing fertilizers (with wood ash, lime, dolomite flour, chalk), as this leads to similar consequences.
Important! "Not recommended" does not mean "not allowed": if you need to add carbamide to acidic soil, then it is advisable to add it with just one of the deoxidizers.
How to improve the effect of fertilizers
- To make urea well absorbed, the soil must berich in organic matter (and urobacteria), in other words, itspreferably applied together with humus or compost.
- If you want to add urea straight into the well when planting seedlings (orin rows when sowing seeds), thentogether with it it is desirable (recommended) to make andpotassium sulfate (potassium sulfate) and / orsuperphosphate.
- Ammonium nitrate should only be entered with superphosphate or without adding other fertilizers at all.
Do these fertilizers cause the accumulation of nitrates in plants?
There is a very popular opinion on the Internet that excessive use of ammonium nitrate can lead to the accumulation of nitrates in vegetables, therefore, it can be taken only in early spring and in no case should the application rate be exceeded. But the use of urea does not lead to the accumulation of nitrates. However, this is not entirely true..
It should be understood that the introduction of any nitrogen fertilizers (ammonium nitrate, urea and even everyone's favorite manure) with excess dosage can lead to the fact that plants will begin to accumulate surplus nitrogen, so to speak, "in reserve" in the form of those same nitrates, which under unfavorable conditions (lack of light, cool weather, lack of molybdenum) turn into a more toxic form - into nitrites, which are extremely dangerous for human health.
Thus, the main thing is comply with the norms of applying nitrogen fertilizers.
Use for spring and autumn eradication spraying (treatment)
- Urea often also used as fungicide and insecticideforeradicating early spring(the very first) andautumn spraying (the very last one) cooking enough concentrated solution (5-7%).
- Ammonium nitrate for such purposes do not apply.
Interesting! Both fertilizers are used for weed control (high concentration simply "burns" them).
Well, now you know which fertilizer suits you best? Perhaps it’s not that simple. As a rule, it is convenient to combine them.
Advice! The site already has detailed material about how and in what doses to use urea and ammonium nitrate for fertilizing and feeding various crops.

